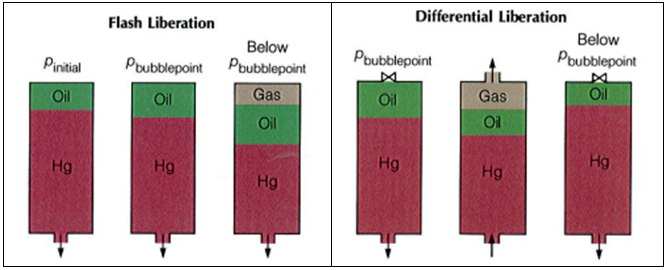
Separator Test experiments are carried out for both oil and gas condensate mixtures. A sample of reservoir liquid is placed in the laboratory cell and brought to reservoir temperature and bubble-point pressure. Then the liquid is expelled from the cell through a number of stages of separation. Usually, two or three stages of separation are used, with the last stage at atmospheric pressure and near-ambient temperature (60 to 80°F).
The gas is let out of the separator through the top and is transferred to standard conditions, where its volume is measured. As for the differential liberation experiment, liquid dropping out from the gas is converted to an equivalent gas volume at standard conditions.
The liquid from the first separator is let into a second separator at a lower pressure and temperature than the first one. At which conditions, more gas will be liberated as sketched in the figure below. As with the gas from the first separator, this gas is transferred to standard conditions.
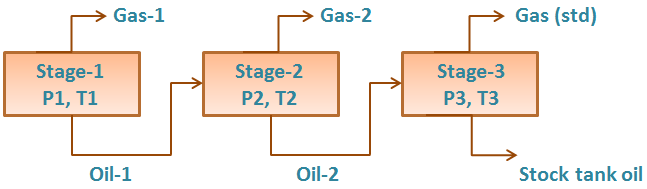
The oil remaining after gas removal is brought to the conditions of the next separator stage. The gas is removed again and quantified by moles and specific gravity. Oil volume is noted, and the process is repeated until stock-tank conditions are reached. Final oil volume, Vo, and specific gravity, SGo, are measured at 60°F.